How Does Dalton's Law Relate to Respiration
Relate daltons law and henrys law to external respiration and internal respiration. How does Daltons law apply in respiration.

Dalton S Law And Henry S Law Youtube
Want to see the full answer.

. Assume that each time you breathe you inhale and exhale about 500 mL. Gas exchange that occurs in the alveoli. Alisa Morgan Katherine Diaz Learning community We breathe in more than 20000 times per day.
As the air warms it expands to a larger volume. Nitrogen 786 percent and oxygen 209 percent. Daltons law of partial pressures defines the partial pressure of a gas in a gas mixture as the pressure that the gas would exert if it occupied the total volume of the mixture in the absence of the other components ie no interactions between gas molecules.
Charles Law does not affect breathing nearly as much as Boyles Law does but it does have an effect. Solution for relate daltons law and henrys law to external respiration and internal respiration. At the air-water interface the amount of gas that dissolves in water is determined by its solubility in water and its partial pressure in the air.
The mechanisms of breathingThe Respiratory Sys. Daltons Law contribution of individual gases to the overall pressure P pGas1 pGas2 pGas3 etc. The only space where Boyles law applies with regard to breathing is the pleural cavity which is enclosed and therefore experiences changes of pressurevolume as the lungs expand and contract.
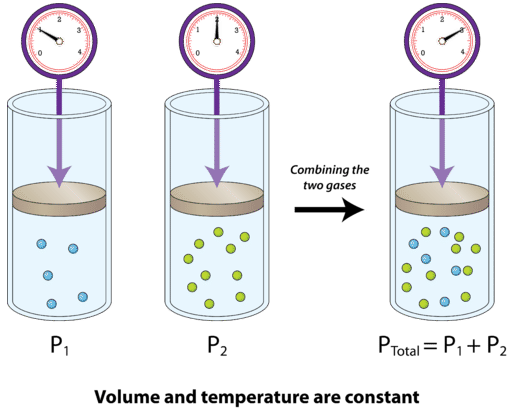
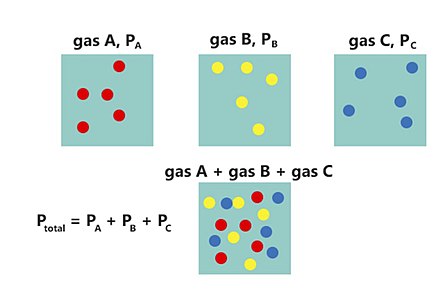
Daltons Law in Respiration Daltons law also implies that the relative concentration of gasses their partial pressures does not change as the pressure and volume of the gas mixture changes so that air inhaled into the lungs will have the same relative concentration of gasses as atmospheric air. The total pressure of a gas mixture is equal to the sum of the partial pressures of its individual gases - Henrys Law. Anatomy Physiology Online from Primal Online LearningBoyles Law - relationship between pressure and volume.
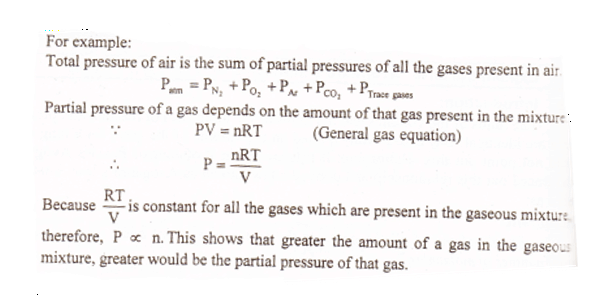
Where p partial pressure - P atmospheric pressure in air - P atmospheric pressure in the alveoli bc there is a different mixture of gases. By increasing the percentage of any gas in airs mixture a higher partial pressure of that gas can be achieved which is the basis of oxygen therapy. Daltons law states that at any given time the percentage of each of these gasses in the air we breathe makes its contribution to total atmospheric pressure and this contribution will depend on how much of each gas is in the air we breathe.
Daltons Law in Respiration. First week only 499. Daltons law statement of the principle that a specific gas type in a mixture exerts its own pressure as if that specific gas type was not part of a mixture of gases external respiration gas exchange that occurs in the alveoli Henrys law.
How does Daltons law relate to respiration. This means that when you inhale cold air it will change volume as it warms in passing through the sinuses. John Dalton 1766-1844 Daltons Law of Partial Pressure Experiment Importance of Respiration The Respiratory System and Daltons Law of Partial Pressure Gas exchange and.
How does Daltons law relate to respiration. Daltons law is used in the practices of pulmonary physiology ventilator care medical gas administration arterial blood gas and pulmonary pathophysiology among other applications. The air you breathe is made predominantly of two gases.
When the properties of gas does not apply the boyles law. Daltons Law in Respiration The air in the atmosphere is a mixture of many different gases that vary in concentration. Check out a sample QA here.
What gas law is P1v1p2v2. Daltons law also implies that the relative concentration of gasses their partial pressures does not change as the pressure and volume of the gas mixture changes so that air inhaled into the lungs will have the same relative concentration of gasses as atmospheric air. At rest the lungs experience fluid flow with an increasingdecreasing volume but as they are open to the static atmosphere there are flowmass changes not.
Give me answer of this question. Daltons Law in Respiration Daltons law also implies that the relative concentration of gasses their partial pressures does not change as the pressure and volume of the gas mixture changes so that air inhaled into the lungs will have the same relative concentration of gasses as atmospheric air. Statement of the principle that a specific gas type in a mixture exerts its own pressure as if that specific gas type was not part of a mixture of gases.
Daltons law states that each of the gases in a gas solution such as air exerts its own pressure based upon its concentration in the solution see Figure 138. Start your trial now.
11 6 Mixtures Of Gases Why Deep Sea Divers Breathe A Mixture Of Helium And Oxygen Chemistry Libretexts
Applications Of Dalton S Law Of Partial Pressure Diffusion And Effusion And Graham S Law Of Diffusion Video Dailymotion

Dalton S Law Of Partial Pressures Dalton S Law Chemistry Respiratory System

What Is The Application Of Dalton S Law Of Partial Pressure Quora

Gas Laws Of Respiratory Physiology Boyle S The Pressure Of A Given Quantity Of Gas Is Inversely Proportional To Physiology Dalton S Law Anatomy And Physiology

05l Respiratory Gas Exchange And Dalton S Law Youtube

Daltons Law Partial Pressures Youtube

Daltons Law Of Partial Pressures Easy Science Dalton S Law Pressure Law Easy Science

Dalton S Law Of Partial Pressures For Anatomy Physiology Youtube

Dalton S Law And Partial Pressures Youtube
Dalton S Law Of Partial Pressure Chemistry Skills

Dalton S Law Of Partial Pressure Urdu Hindi Class 11 Chemistry State Of Matter Gaseous State Youtube

Dalton S Law Of Partial Pressures For Anatomy Physiology Youtube

8 8 Partial Pressure Dalton S Law Ppt Download

The Respiratory System And Dalton S Law Of Partial Pressure By Alisa Morgan
Dalton S Law Of Partial Pressures Dalton S Law Of Partial Pressures Estimation Applications Of Dalton S Law


Comments
Post a Comment